The Roles of Cilia in Brain Development and Tumor Formation
The mysterious organelle "primary cilium"
in neural stem cells plays distinct roles in different stages
during development. Ciliary dysfunctions in human (i.e., ciliopathy)
cause developmental defects in multiple organs, including
brain developmental delays, which lead to intellectual disabilities
and cognitive deficits. However, effective treatment to this
devastating developmental disorder is still lacking. Here
we first investigated the effects of ciliopathy on neural
stem cells by knocking down Kif3a, a kinesin II motor required
for ciliogenesis, in the neurogenic stage of cortical development
by in utero electroporation of mouse embryos. Brains electroporated
with Kif3a shRNA showed defects in neuronal migration and
differentiation, delays in neural stem cell cycle progression,
and failures in interkinetic nuclear migration. Interestingly,
introduction of Gli2, but not Gli1, restored the ability of
cilium-deficient neurons to differentiate and move from the
germinal ventricular zone (VZ) to the cortical plate. Moreover,
Cyclin D1 knockdown abolished Gli2's rescue effect. These
findings suggest Gli2 may rescue neural stem cell proliferation,
differentiation and migration through Cyclin D1 pathway. This
work, published in the leading journal in the field of brain
development Cerebral Cortex, showed that Gli2
may serve as a potential therapeutic target for human ciliopathy
syndromes through modulating the progression of neural stem
cell cycle.
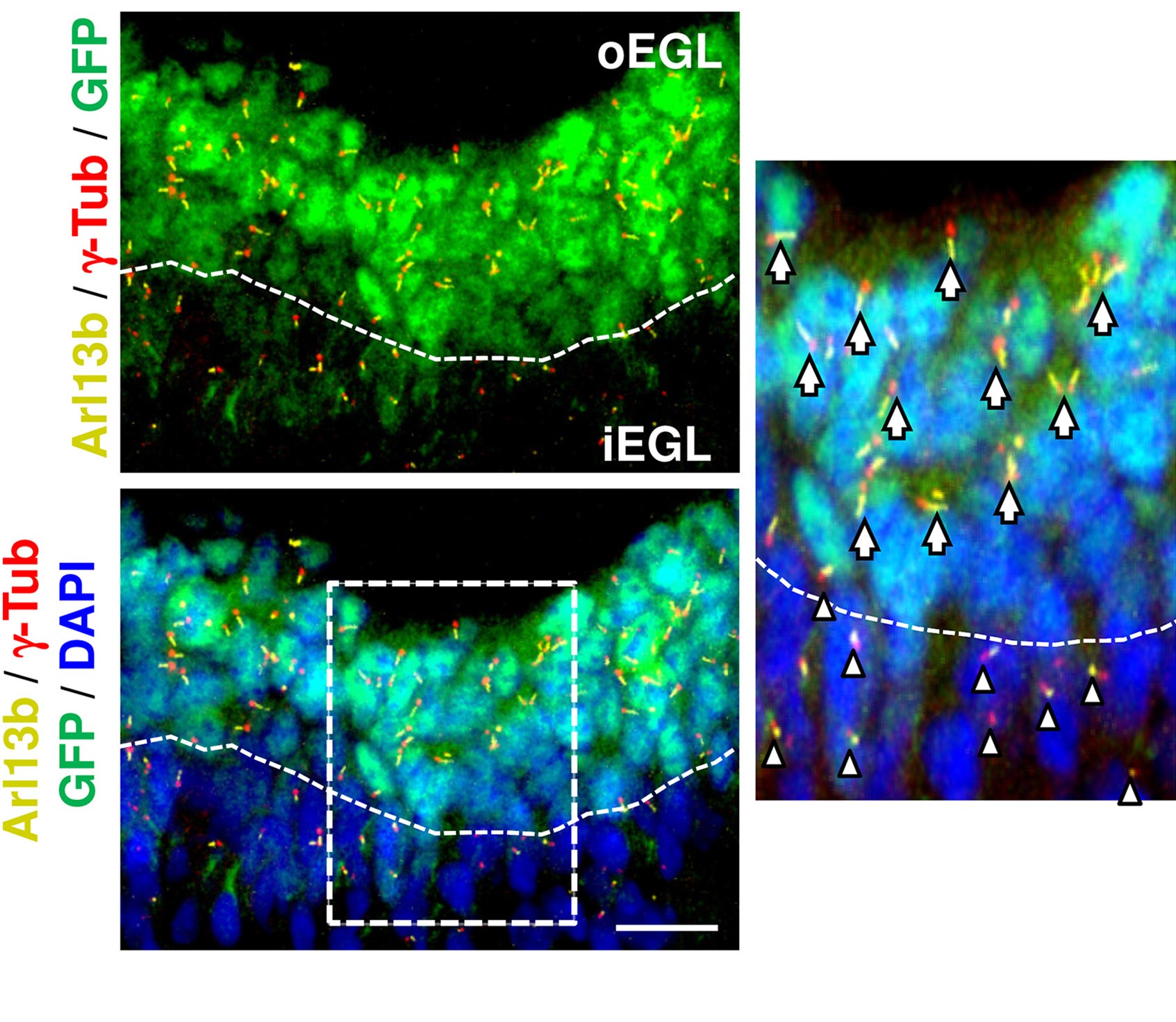
We
also studied the role of primary cilia in the proliferation
of cerebellar granule neuron progenitors (GNPs) in collaboration
of Dr. Olivier Ayrault at Curie Institute, France. Development
of the cerebellum requires the primary cilium to allow the
transduction of Sonic Hedgehog (SHH) signaling. Besides, precise
regulation of ciliogenesis ensures the proliferation of cerebellar
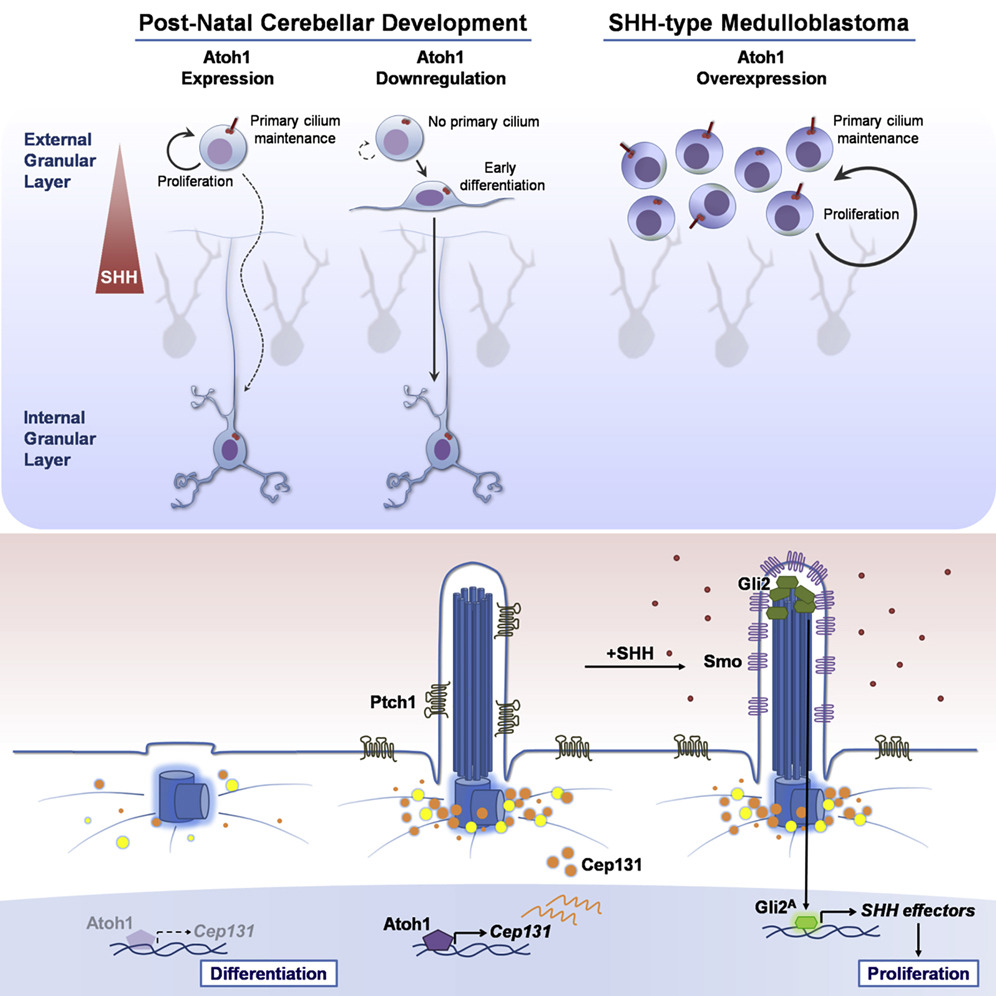
granule neuron progenitors (GNPs). Here, we report that Atoh1,
a transcription factor required for GNPs formation, controls
the presence of primary cilia, maintaining GNPs responsive
to the mitogen SHH. Loss of primary cilia abolishes the ability
of Atoh1 to keep GNPs in proliferation. Mechanistically, Atoh1
promotes ciliogenesis by transcriptionally regulating Cep131,
which facilitates centriolar satellite (CS) clustering to
the basal body. Importantly, ectopic expression of Cep131
counteracts the effects of Atoh1 loss in GNPs by restoring
proper localization of CS and ciliogenesis. This Atoh1-CS-primary
cilium-SHH pro-proliferative pathway is also conserved in
SHH-type medulloblastoma, a pediatric brain tumor arising
from the GNPs. Together, our data reveal the mechanism whereby
Atoh1 modulates the primary cilium functions to regulate GNP
development. The related articles are recently published in
Developmental Cell and Journal of Cell
Science, etc.
Further Readings:
- Chang
CH, Zanini M, Shirvani H, Cheng JS, Yu H, Feng CH, Mercier
AL, Hung SY, Forget A, Wang CH, Cigna SM, Lu IL, Chen WY,
Leboucher S, Wang WJ, Ruat M, Spassky N, Tsai JW*, Ayrault
O* (2019) Atoh1
requires primary cilia for the expansion of granule neuron
progenitors by modulating centriolar satellites. Dev
Cell, 48(2):184-199.e5.
- Chen
JL, Chang CH, Tsai JW* (2019) Gli2
rescues delays in brain development induced by Kif3a dysfunction.
Cereb Cortex, 29(2):751-64.
- Hsiao
CJ, Chang CH, Ibrahim RB, Lin IH, Wang CH, Wang WJ, and
Tsai JW* (2018) Gli2
modulates cell cycle re-entry through autophagy-mediated
regulation on the length of primary cilia. J Cell
Sci, 131(24). pii: jcs221218.
Last
updated 6/13/2013. Copyright© 2013
Jin-Wu Tsai. All rights reserved.
|
