Neural
Stem Cell Research
A
new subtype of neural progenitor
|
One
of the most notable features in the evolution of the
neocortex is the increase in neuron number that reaches
its peak in the human brain. Although the laminar organization
of the cortex is relatively similar in all mammals,
an expansion in cortical surface area underlies the
transformation from smooth cortex to the highly folded
primate neocortex, and the associated alteration of
cortical architecture that is the substrate for the
'higher' cortical functions that distinguish human from
other species. This transition underscores the importance
of understanding the process of neurogenesis in the
developing neocortex.
Recent
studies have identified two subtypes of neuronal progenitor
cell in the developing rodent embryonic neocortex: radial
glia (RG) and intermediate progenitors (IP). Neuroepithelial
cells located in the apical-most region, the ventricular
zone, transform to RG cells at the onset of neurogenesis.
In addition to their well characterized function as
a scaffold supporting neuronal migration, RG constitute
the main population of neural progenitor cells in the
developing mammalian neocortex.
An
evolutionary increase in size and functional complexity
of the cerebral cortex has culminated in the modern
human brain, which diverged from a rodent lineage ~100
million years ago. Recent studies suggest that the development
of oRG cells and their transit-amplifying daughter cells
(that is, intermediate progenitor¡Vlike cells)
may be the cellular mechanism underlying expansion in
primate corticogenesis. Recently in the fetal human
cortical tissue, a new subtype of neural progenitor
cells, termed the oRG (outer subventricular zone-RG)
cells, with radial glia¡Vlike morphology but lacking
apical processes was discovered. oRG cells can self-renew
and produce neuronal precursors. It has been suggested
that the outer subventricular zone (OSVZ) may be a primate-specific
feature and a hallmark of primate corticogenesis. Although
the radial glia cells and intermediate progenitor cells
of the ventricular zone and SVZ, respectively, are responsible
for generating most cortical neurons in rodent, extra
sites of progenitor cell activity have been suggested,
which prompted us to ask whether oRG-like cells exist
in the developing mouse neocortex.
To
address these issues, we investigated whether progenitor
cells resembling oRG cells exist in the rodent brain
during periods of neocortical neurogenesis. We found
cells in the superficial region of the subventricular
zone (SVZ) in the developing mouse cortex that morphologically
resembled oRG cells. Time-lapse imaging revealed that
these cells underwent "mitotic somal translocation"
and asymmetric division in which one daughter cell inherited
the basal process. Our long-term imaging revealed that
oRG cells were generated directly from radial glia cells
and that they produced neurons directly, without an
intervening intermediate progenitor cell. Furthermore,
we found that during interphase, the centrosome moved
into the basal process to maintain polarity before mitotic
somal translocation. These results suggest that oRG
cells are not a specialization of a larger brain with
greater cortical area. Instead, oRG-like cells are probably
present in all mammals, and an evolutionary increase
in the number of oRG cells likely amplified neuronal
production and contributed to cortical expansion.
|
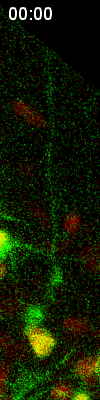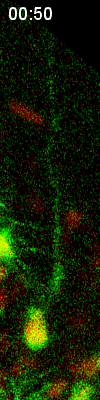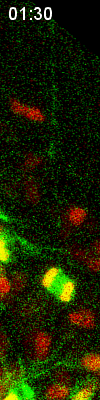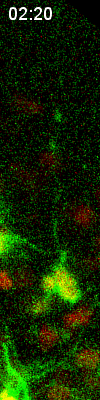
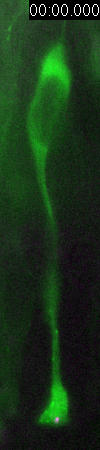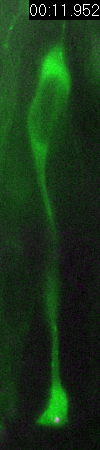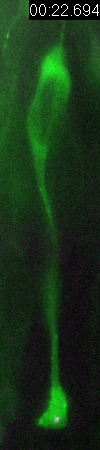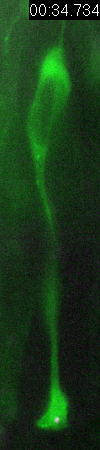
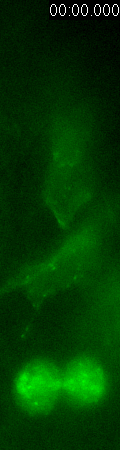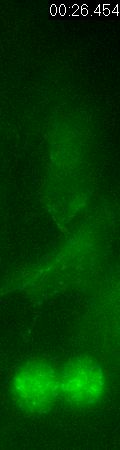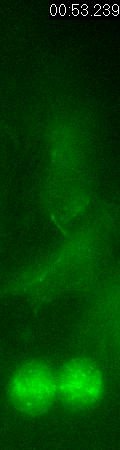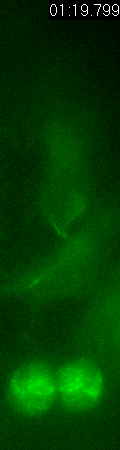
Cell
division of an ORG progenitor
Nature
Neuroscience features the finding of the new subtype
of neural progenitor
|
Molecular
mechanism of "interkinetic nulcear migration" in
neural stem cells
|
Neocortical
neurons are born in the germinal zone of the developing
mammalian brain and migrate over substantial distances
to the forming cortical layers. The mechanisms that
are involved in the initial stages of neocortical neurogenesis
are not well understood. Neuroepithelial cells, referred
to as radial glial progenitor cells (RGPCs) as the neocortex
thickens, divide rapidly to expand their pool and undergo
asymmetric divisions to generate most cortical pyramidal
neurons and glia. Each progenitor spans the entire thickness
of the neural tube and developing neocortex and shows
an unusual behavior termed interkinetic nuclear migration
(INM). After mitosis, which occurs exclusively at the
ventricular surface, the nuclei ascend to the upper
region of the ventricular zone, where they undergo S
phase, and then descend back to the ventricular surface.
This behavior is seen in most neuroepithelial cells
in the CNS and in some polarized non-neuronal cells.
Although
INM was described in the early part of the twentieth
century, little was known until recently about its biological
significance, its role in neurogenesis and its underlying
mechanism. We
carried out a detailed analysis of nuclear migration
and microtubule organization in RGPCs and evaluated
the contributions of microtubule- and actin-based motors
to INM. We found that dynein was required for apical,
but not basal, migration, which instead required an
unconventional kinesin. Nuclear movement was independent
of centrosome behavior and occurred along an array of
uniformly oriented microtubules that span the entire
length of the progenitor cell. Unlike others, we did
not find any effect of inhibition of myosin II in our
system. These results lead to a model in which INM is
powered by oppositely directed microtubule motors that
are regulated in a cell cycle¡Vdependent manner.
|
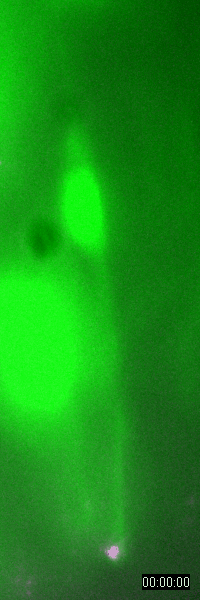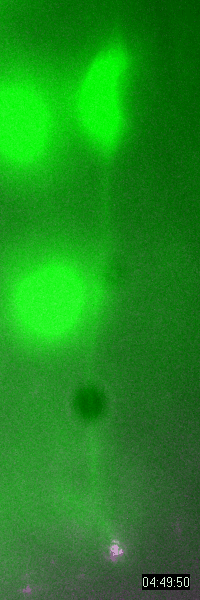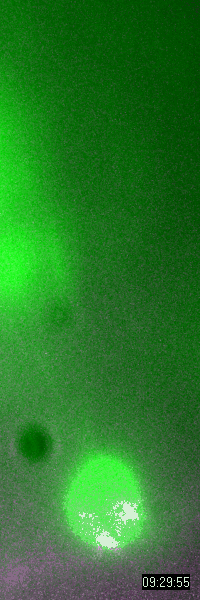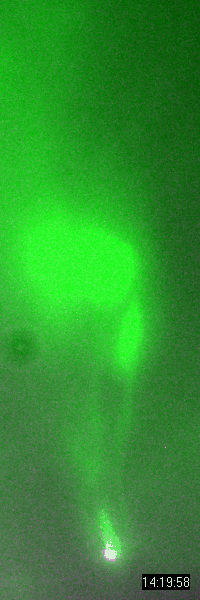
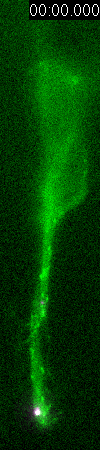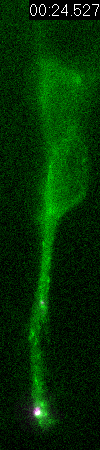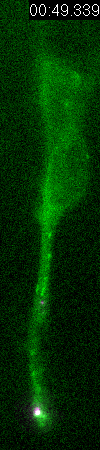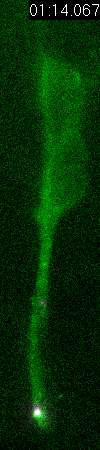
Behavior
of a neural progenitor (RG) cell (green) and its centrosome
(magenta)
|
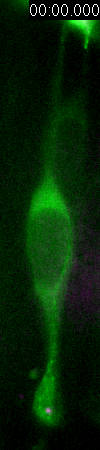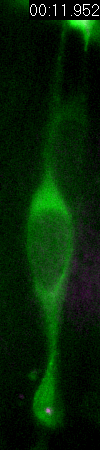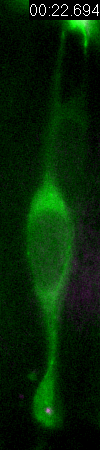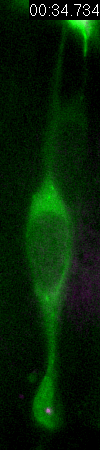

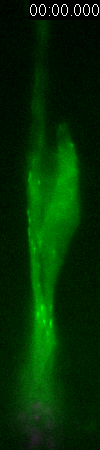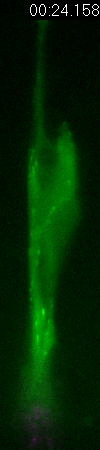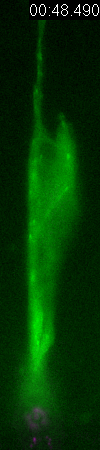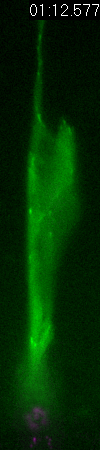
GFP-EB3
behavior and microtubule organization in radial glial cells
at different cell-cycle stages. The EB3 streaks (green)
represent the growing end of individual microtubules. When
the soma is in the top of the ventricular zone (G2), EB3 streaks
mostly originate from the centrosomal region in the endfeet,
curve around the nucleus and enter the basal process (left
two panels). During mitosis (M), EB3 streaks radiate from
the two spindle poles to form the mitotic spindle. No detectable
EB3 streaks enter the basal process (3rd panel). During cytokinesis,
the microtubules radiate from the centrosomes in each daughter
cell, with many microtubules aimed toward the midbody. EB3
streaks remain absent from the basal processes (4th panel).
Non-radial glial cells are seen in upper image. In G1 phase
(5th panel), paired cells after probable symmetric cell division
with centrosomes at the endfeet of both daughter cells. EB3-tipped
microtubules are oriented upward in both cells and re-enter
the basal fibers. In another case (rightmost panel), paired
cells after probable asymmetric cell division. The centrosome
of daughter cell at right is shifted away with EB3 streaks
emerging radially to form a bidirectional microtubule array.
Asymmetric
inheritance of mother and daughter centrosomes in nerual stem
cell division
|
Radial
glia cells constitute a major population of neural progenitor
cells that occupy the proliferative VZ in the developing
mammalian neocortex. In addition to their well-characterized
function as a scaffold in supporting neuronal migration,
radial glia cells display interkinetic nuclear oscillation
and proliferate extensively at the luminal surface of
the VZ. During the peak phase of neurogenesis, they
predominantly undergo asymmetric division to self-renew
while simultaneously giving rise either directly to
a neuron, or to an intermediate progenitor cell which
subsequently divides symmetrically to produce neurons.
Whereas differentiating progeny progressively migrate
away from the VZ to form the cortical plate (CP)¡Xthe
future neocortex¡Xrenewing radial glia progenitors
remain in the VZ for subsequent divisions. The distinct
migratory behaviour of radial glia progenitors and their
differentiating progeny is fundamental to the proper
development of the mammalian neocortex; however, little
is known about the basis of these behavioural differences.
Centrosomes,
the main microtubule-organizing centres in animal cells,
have an important role in many cell processes, particularly
during cell division10 and cell migration. All normal
animal cells initially inherit one centrosome, consisting
of a pair of centrioles surrounded by an amorphous pericentriolar
material. The two centrioles differ in their structure
and function. The older ¡¥mother¡¦
centriole, which is formed at least one-and-a-half generations
earlier, possesses appendages/satellites that bear specific
proteins, such as cenexin (also known as Odf2) and ninein,
and anchor microtubules and support ciliogenesis. In
contrast, the younger ¡¥daughter¡¦
centriole, which is formed during the preceding S phase,
lacks these structures. Full acquisition of appendages/satellites
by the daughter centriole is not achieved until at least
one-and-a-half cell cycles later. During each cell cycle,
the centrosome replicates once in a semi-conservative
manner, resulting in the formation of two centrosomes:
one of which retains the original old mother centriole
(that is, the mother centrosome) while the other receives
the new mother centriole (that is, the daughter centrosome).
This intrinsic asymmetry in the centrosome has recently
been demonstrated to be important for proper spindle
orientation during the division of male germline stem
cells and neuroblasts in Drosophila, although
female germline stem cells appear to divide normally
in the absence of centrioles/centrosomes. These studies
indicate a critical role for the differential behaviour
of centrosomes with differently aged mother centrioles
in asymmetric division of the progenitor/stem cells,
although it remains unclear whether proper behaviour
and development of the progenitor/stem cells and their
differentiating daughter cells depend on centrosome
asymmetry.
Asymmetric
division of radial glia progenitors accounts for nearly
all neurogenesis in the developing mammalian neocortex.
Three out of four autosomal recessive primary microcephaly
(MCPH) genes identified so far encode centrosomal components,
suggesting that proper neocortical neurogenesis and
development entail a tight regulation of the centrosome,
which is poorly understood. In collaboration with Dr.
Songhai Shi's lab, we investigated centrosome regulation
during the peak phase of mammalian neocortical neurogenesis.
We show that asymmetric centrosome inheritance regulates
the differential behaviour of renewing progenitors and
their differentiating progeny in the embryonic mouse
neocortex. Centrosome duplication in dividing radial
glia progenitors generates a pair of centrosomes with
differently aged mother centrioles. During peak phases
of neurogenesis, the centrosome retaining the old mother
centriole stays in the VZ and is preferentially inherited
by radial glia progenitors, whereas the centrosome containing
the new mother centriole mostly leaves the VZ and is
largely associated with differentiating cells. Removal
of ninein, a mature centriole-specific protein, disrupts
the asymmetric segregation and inheritance of the centrosome
and causes premature depletion of progenitors from the
VZ. These results indicate that preferential inheritance
of the centrosome with the mature older mother centriole
is required for maintaining radial glia progenitors
in the developing mammalian neocortex.
|
Nature
magzine features the story of asymmetric centrosome
inheritance in nerual stem cell division

Asymmetric
inheritance of the mother (red/green) and daughter (green)
centrosomes
|
Source
and further readings:
Links:
Last
updated 6/13/2013
|
