Cellular
and Molecular Mechanism of Neuronal Migration
美國哥倫比亞大學(Columbia
University)新聞報導我們的發現...>
HOT!!!
| 腦皮質(cerebral cortex)在發育過程中,位於腦室區(ventricular
zone)之神經幹細胞(radial glial cell)分裂生成新生的神經細胞,新生的神經細胞沿著radial
glia fiber遷移,往外層皮質移動並成熟,進而發展出結構分明的大腦皮質。當神經細胞遷移時,前導突起(leading
process)首先膨大,中心粒接著進入膨大處,再帶動細胞本體,最後細胞後端收縮推動細胞核 (下圖)。這個中心粒離開細胞本體的過程非常特殊,因此其機制非常值得探討。許多研究指出,若此過程發生任何差錯,則皮質結構將產生問題而導致嚴重的腦部發育疾病,例如平腦症(lissencephaly)乃由LIS1基因突變所造成,我們發現LIS1功能喪失後,神經細胞的中心粒和細胞核均無法遷移,導致神經細胞無法遷移,進而造成大腦表面平滑的發育缺陷
(Tsai
et al., 2005)。
神經細胞遷移如此重要,但其機制至今卻仍未闡明。神經細胞移動時,細胞膜上的黏著蛋白與細胞外間質(extracellular
matrix)產生交互作用,當神經細胞黏著後,細胞膜內的蛋白質和細胞骨架的交互作用使細胞往前遷移 (Vallee
et al., 2009)。在這個過程中的物理特性為何,中心粒、細胞核、細胞骨架相互間之交互作用等過程目前仍有許多值得探討的地方。
Cells
make use of a variety of mechanisms for directed migration.
Recent attention has been directed at the unusual migratory
behavior of a form of stem cell - the neural precursor.
These cells, located at the surface of the ventricles
in the developing brain, give rise to all neuronal and
glial cells in the developing brain. They undergo numerous
successive cycles of division to populate the forming
cerebral cortex, the part of the brain responsible for
cognitive function. As new cells are produced they migrate
outward over considerable distances to find their proper
location in the developing brain. Defects in the division
of these cells can lead to microencephaly, or "small
brain," and defects in migration can lead to a
number of brain developmental disorders e.g., lissencephaly
(smooth brain), double cortex, and periventricular heterotopia.
However, how neuronal migration affects brain development
and how defects in this process cause human developmental
diseases in newborn infants were largely unknown.
Depending
on their site of origin, neural precursors may pass
through a series of morphological stages, but in each
case long-distance migration involves a specific cell
form which exhibits behavior not seen in the migration
of non-neural cells. Existing evidence suggests that,
unlike most cell types, newborn neuron moves in a strikingly
discontinuous, or saltatory, manner. In these events,
subcellular structures such as the cytoskeleton ("bones"
of cells), the nucleus (which contains the genetic material,
DNA) and other organelles ("organs" of cells)
must move in a specific sequence. However, the molecular
mechanisms which these organelles utilize are still
unclear and the genes that are involved are largely
unexplored.
Emerging
evidence has indicated that the lissencephaly gene LIS1
play an essential role in neuronal migration. Previously,
by live imaging of neural precursor cells in brain slices
Dr. Tsai has shown that deficiencies in the LIS1 gene
cause abnormalities in neuronal redistribution. These
results indicated that LIS1, and presumably its regulatory
target cytoplasmic dynein (a molecular motor protein),
were responsible for coupling cell body movement to
migration. The stage of migration in which LIS1 and
cytoplasmic dynein participate is uncertain.
Our
studies aim to define the subcellular events involved
in neural precursor cell migration and to define the
roles of dynein and LIS1 in this process. In order to
elucidate the mechanism of neuronal migration and its
role in causing braining developmental diseases, we
used advanced molecular technologies to fluorescently
label the cytoskeleton, the nucleus and other organelles
in neuronal cells and monitored of the behavior of these
subcellular structures in migrating neurons across live
brain tissue. This research reveals a variety of novel
cell behaviors and provides the first-time demonstration
of the subcellular behavior of neural progenitors in
the live developing brain.
We
foundthat centrosomes often moved far in advance of
nuclei, which exhibited extreme saltatory behavior.
Inhibition of LIS1 and dynein blocked both centrosomal
and nuclear movement. To gain insight into the underlying
mechanisms for dynein-mediated movement, we made the
first use of live microtubule imaging in living brain
tissue, and observed a clear, centrosome-centered radial
arrangement which persists throughout the migration
cycle. In contrast to other undifferentiated cells,
the distribution of neural precursor microtubules is
markedly constrained by the narrow neural processes.
By immunocytochemistry, we found a pronounced accumulation
of dynein within the migratory process correlated with
the onset of centrosomal movement. All these results
have brought about a number of striking and unique features
to the underlying organization and migration of neural
progenitor cells, and led to a comprehensive model for
how microtubule, nucleus, and dynein behavior are coordinated
to affect the complex temporal and morphogenetic behavior
of these cells.
|

Motile
behavior of the centrosome (green) and the nucleus (magenta)
in a migrating neuron (blue) during brain development.
|
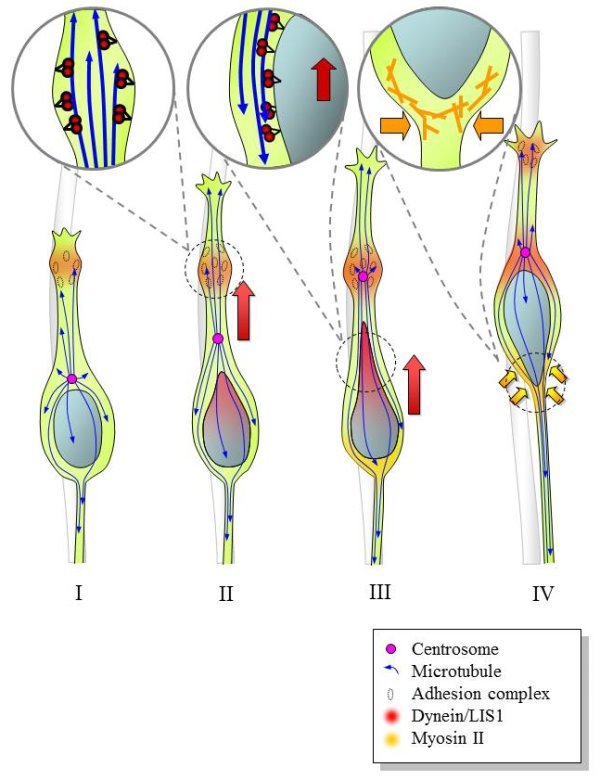
Schematic
diagram depicting mechanism underlying neural precursor migration.
Swellings form within the migratory process possibly at sites
of adhesion to the radial glial fiber (I). Dynein is recruited
to the swelling, from which site it pulls on the plus ends
of microtubules, resulting in forward movement of the centrosome
and, presumably, the entire microtubule network (II). The
trailing microtubules engage the dynein on the nuclear surface
and pull the nucleus forward (III). At the same time, myosin
II contracts from the rear of the soma, where it may participate
in proximal axon formation and in forward nuclear movement
(IV).
Further
readings:
- Vallee
RB, Seale GE, Tsai JW. Emerging roles for myosin II and
cytoplasmic dynein in migrating neurons and growth cones.
Trends
in Cell Biology. 2009 Jul;19(7):347-55.
- Tsai
MH, Cheng HY, Nian FS, Liu C, Chao NH, Chiang KL, Chen SF,
Tsai JW* (2020) Impairment
in Dynein-Mediated Nuclear Translocation by BICD2 C-terminal
Truncation Leads to Neuronal Migration Defect and Human
Brain Malformation. Acta Neuropathol Commun,
8(1):106. (* corresponding)
- Jheng
GW, Hur SS, Chang CM, Wu CC, Cheng JS, Lee HH, Chung BC,
Wang YK, Lin KH, del Alamo JC, Chien S, Tsai JW*
(2018). Lis1
dysfunction leads to traction force reduction and cytoskeletal
disorganization during cell migration. Biochem
Biophys Res Commun, 497, 869-75.
- Chen
YA, Lu IL, Tsai JW* (2018) Contactin-1/F3
regulates neuronal migration and morphogenesis through modulating
RhoA activity. Front Mol Neurosci, 11:422.
- Chen
HR, Juan HC, Wong YH, Tsai JW, Fann MJ (2017) Cdk12
regulates neurogenesis and late-arising neuronal migration
in the developing cerebral cortex. Cereb Cortex,
27:2289-302.
- Liu
YH, Tsai JW, Chen JL, Yang WS, Chang PC, Cheng PL,
Turner DL, Yanagawa Y, Wang TW, Yu JY (2017) Ascl1
promotes tangential migration and confines migratory routes
by induction of Ephb2 in the telencephalon. Sci
Rep, 7, 42895.
Last
updated 6/13/2013. Copyright© 2013 Jin-Wu
Tsai. All rights reserved.
|
