雙光子顯微鏡
Two-Photon In
Vivo Microscopy
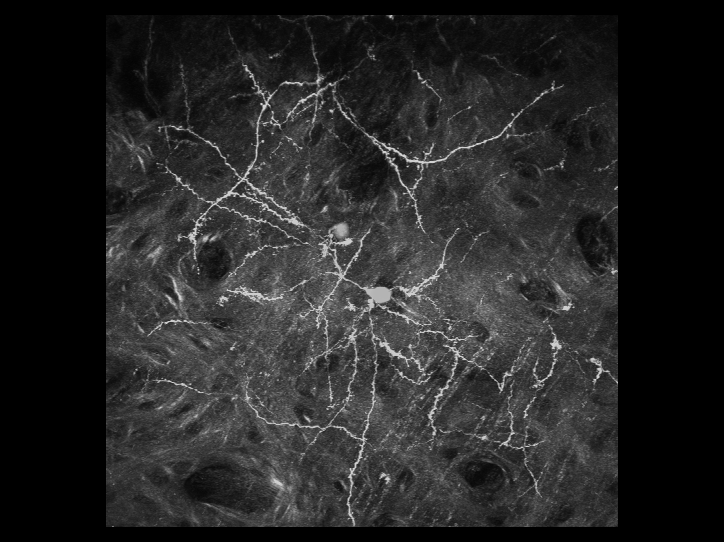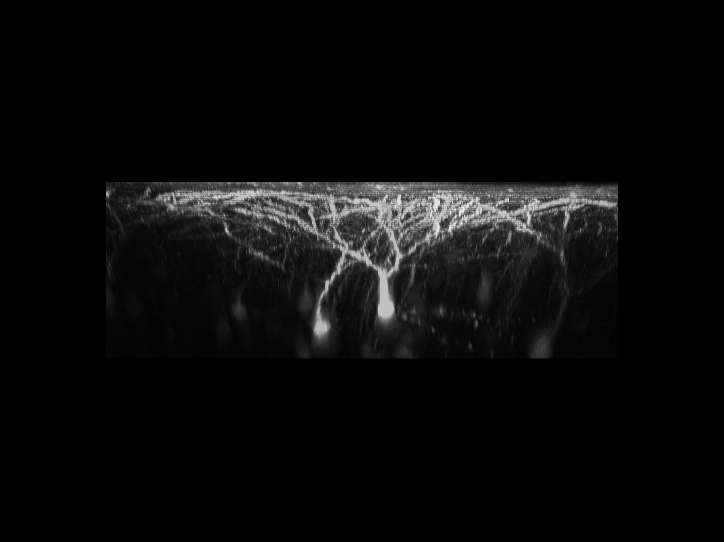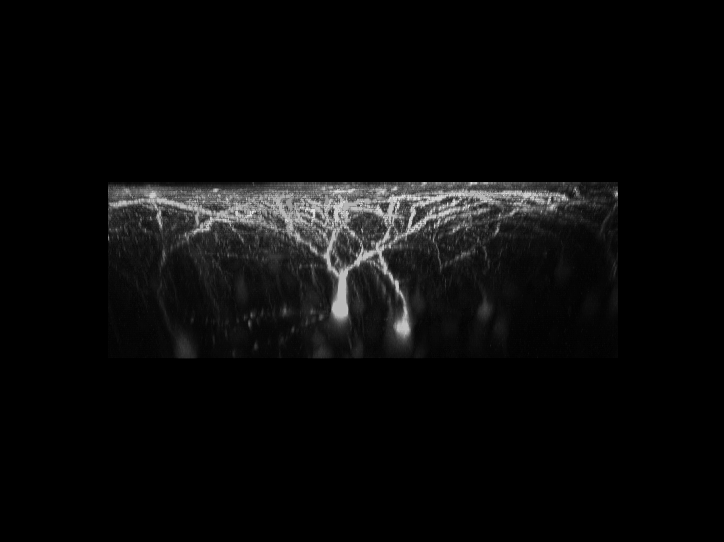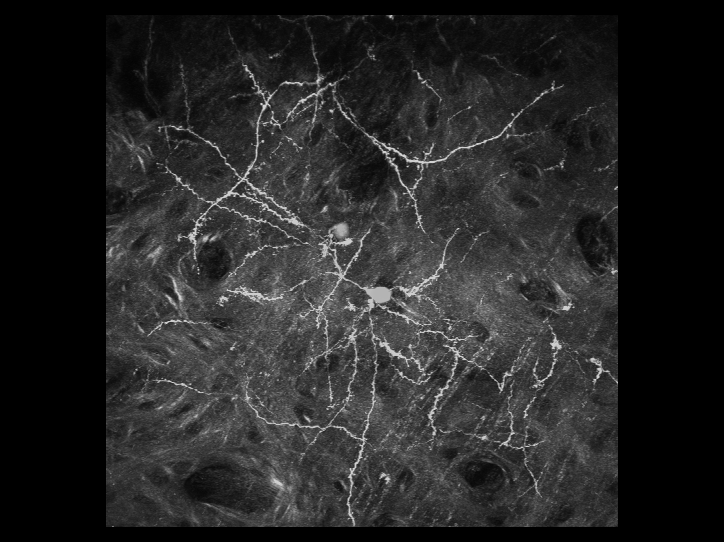
利用雙光子顯微鏡,可以用來觀察活體生物深層組織的細胞與結構。我們對於最先進的顯微鏡系統有豐富的使用者經驗
。實驗室擁有改良後的腦殼削薄技術,因此更利於長時間觀察活體小鼠大腦中神經細胞的型態,且此技術不會導致神經細胞的發炎而影響結果。利用此技術,可用3D影像重新勾勒出位於大腦皮質之下的螢光標記神經元的型態。且可連續觀察神經元樹突和突觸的動態影像數個星期,甚至是長達一個月。
Following
brain injury, injured neurites usually cannot regenerate past
the lesion. The failure of neural regeneration is at least
partially due to the "scar" that forms at the injury
site. The scar is formed as a result of a series of cellular
and molecular events, which occurs over days to weeks upon
injury. The major cell types involved in the scar formation
include microglia, macrophages, oligodendrocyte precursors,
meningeal cells and astrocytes. Resident micoglia and macrophages
from the blood stream are the first cells to arrive at the
injury site, within hours of injury. The final structure of
the scar mainly consists of astrocytic processes and microglia.
Many molecules, such as transforming growth factor b
(TGF-b), interleukin 1, and basic
fibroblast growth factors, have been implicated as mediators
of glial scar formation. Although glial scar may be important
for restoring a stable environment for the neurons when injury
or local bleeding occurs, it presents a physical barrier to
regenerating neurites. Furthermore, many molecules in the
scar, including chondroitin sulfate proteoglycans and tenascins,
prevent neural regeneration. Despite the important role of
microglial cells in various pathological situations such as
brain injury, Alzheimer's disease and stroke, there has been
no direct observation of microglial accumulation at lesions
in spinal cord injury and the relationship between microglial
accumulation and neural regeneration remains unclear. Therefore,
it is essential to understand the sequence of events leading
to scar formation and to prevent or even reverse the detrimental
effects of the scar on the regenerating neurites.
Recently,
we have achieved major advances in the investigation of the
dynamic properties of microglia and the mechanisms underlying
their response upon injury in the living animals by taking
advantage of transgenic mice in which all microglia are fluorescently
labeled by homologous recombination in embryonic stem cells.
Using intravital two-photon laser scanning microscopy, the
behavior of fluorescent microglia under normal and traumatic
injury conditions can be readily imaged in the cerebral cortex
of living mice. Using this unique model system, we are now
monitoring microglial migration and accumulation at lesions
after brain injury and stroke. We have been dedicated to the
further determination of the possibility of inducing microglia
migration away from glial scar. Our studies can not only provide
a first glimpse at microglial response, scar formation, and
neural regeneration following brain injury and test treatments
aimed at enhancing neural regeneration, but also form a solid
basis for future testing of other therapeutic strategies.
- Chakraborty
S, Karmenyan A, Tsai JW, Chiou A (2017) Inhibitory effects
of curcumin and cyclocurcumin in 1-methyl-4-phenylpyridinium
(MPP+) induced neurotoxicity in differentiated PC12 cells.
Sci Rep, 7, 16977.
- Ma
L*, Qiao Q*, Tsai JW*, Yang G, Li W, Gan WB (2016) Experience-dependent
plasticity of dendritic spines of layer 2/3 pyramidal neurons
in the mouse cortex. Dev Neurobiol, 76, 277-286. (*equal
contribution)
- Qiao
Q, Ma L, Li W, Tsai JW, Yang G, Gan WB (2016) Long-term
stability of axonal boutons in the mouse barrel cortex.
Dev Neurobiol, 76, 252-261.
- Chakraborty
S, Nian FS, Tsai JW, Karmenyan A, Chiou A (2016) Quantification
of the metabolic state in cell-model of Parkinson's disease
by fluorescence lifetime imaging microscopy. Sci Rep, 6,
19145.
Links:
Last
updated 6/13/2013. Copyright© 2013
Jin-Wu Tsai. All rights reserved.
|
